Prescribing PROMACTA

EFFICACY & SAFETY
Rapid response: As early as Week 11,2
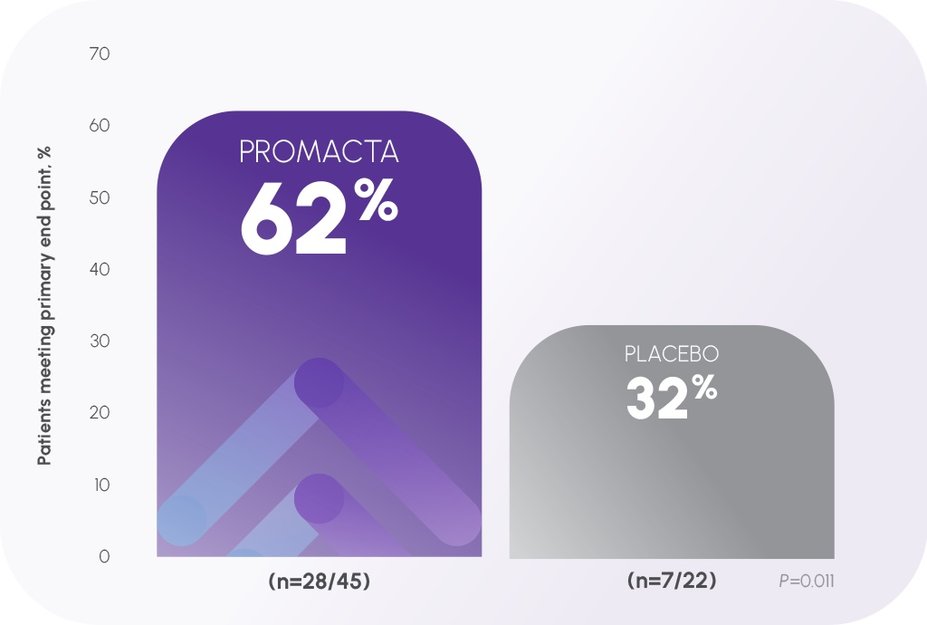
Platelet levels ≥50,000/mcL (PETIT)1,2
In the PETIT pivotal trial:
Primary end point: The proportion of patients who achieved a response (defined as platelet count ≥50,000/mcL in absence of rescue therapy) at least once between Weeks 1 and 6 of the randomized, double-blind period1,2
At baseline, ~51% of patients had platelet counts ≤15,000/mcL1
At Week 1, 24% of patients receiving PROMACTA achieved platelet levels ≥50,000/mcL vs 14% with placebo2
Study Design
The PETIT trial was a phase 2, 7-week, double-blind trial in children ≥1 year of age with relapsed or refractory ITP, followed by an open-label period of up to 24 weeks when patients from both arms were eligible to receive PROMACTA.1,2 Sixty-seven patients were randomized 2:1 to PROMACTA (n=45) or placebo (n=22) and permitted to use maintenance therapy, including (but not limited to) steroids, azathioprine, danazol, CsA, and mycophenolate mofetil.1,2 Platelet response was evaluated overall and for 3 age cohorts: 1) 12 to 17 years, 2) 6 to 11 years, and 3) 1 to 5 years.1
Durable response: Levels maintained for ≥6 weeks1,3,*
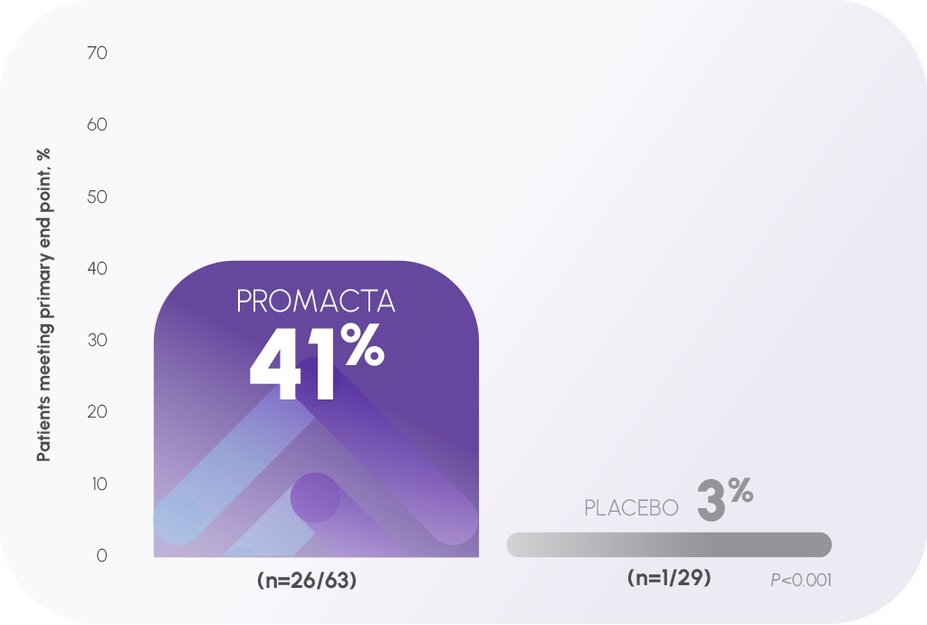
Platelet levels ≥50,000/mcL (PETIT 2)1,3
In the PETIT 2 pivotal trial:
- Primary end point: The proportion of patients who achieved a response (defined as platelet count ≥50,000/mcL in absence of rescue therapy) for at least 6 of the 8 weeks between Weeks 5 and 12 of the randomized, double-blind period1,3
- At baseline, ~62% of patients had platelet counts ≤15,000/mcL1
Study Design
The PETIT 2 trial was a phase 3, 13-week, double-blind trial to assess the efficacy and safety of PROMACTA in children ≥1 year of age with relapsed or refractory ITP, followed by a 24-week open-label extension phase when patients from both arms were eligible to receive PROMACTA.1,3 Ninety-two patients were randomized 2:1 to PROMACTA (n=63) or placebo (n=29) and permitted to use maintenance therapy, including steroids, IVIg, CsA, mycophenolate, azathioprine, and dapsone.1,3 Patients were permitted to reduce or discontinue baseline ITP therapy only during the open-label phase of the trial. Platelet response was evaluated overall and for 3 age cohorts: 1) 12 to 17 years, 2) 6 to 11 years, and 3) 1 to 5 years.1
*In the extension phase of the study, platelet levels ≥50,000/mcL were maintained for a mean of 8.6 weeks and a median of 6 weeks.3
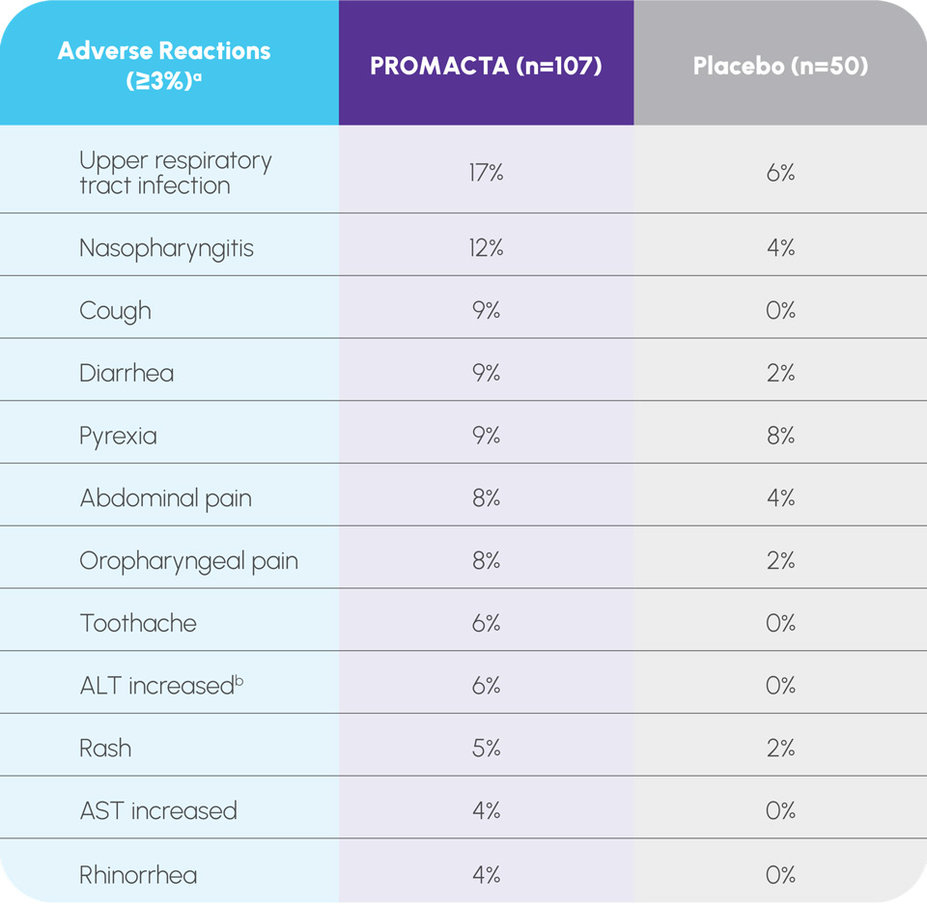
Safety established in 2 pediatric trials with patients aged 1 year and older1
Pooled results from PETIT and PETIT 21
aThe adverse events listed above were pooled from the placebo-controlled phases of the PETIT and PETIT 2 clinical trials.
bIncludes adverse reactions or laboratory abnormalities 3 × ULN.
During the randomized period of PETIT: 6.8% of patients experienced grade 3 and 4.5% of patients experienced grade 4 adverse events during the randomized period2
During the randomized period of PETIT 2: 12.7% of patients experienced grade 3 and no patients experienced grade 4 adverse events during the randomized period3
DOSING & ADMINISTRATION
Convenient once-daily oral dosing: At home, at school, or on the go1
PROMACTA can be taken without food or with food low in calcium (≤50 mg)
PROMACTA should be taken at least 2 hours before or 4 hours after medications such as antacids and mineral supplements or foods high in calcium
PROMACTA can be taken any time of day, at the same time each day
No need for weekly office visits for injections and with PROMACTA tablets, there is less unused medication to discard
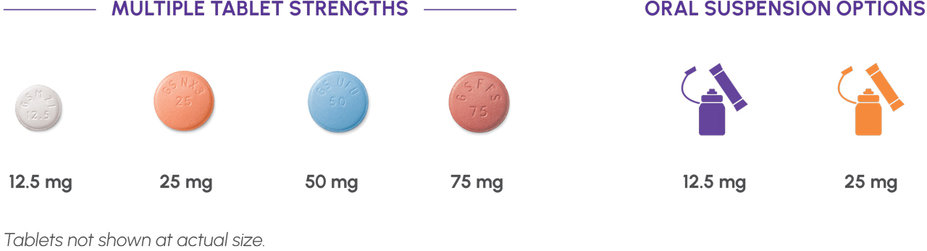
Two oral formulations: Dosing flexibility, even for patients who have difficulty swallowing a pill1
Recommended starting dose for patients with persistent or chronic ITP1
a Except in patients who are of East-/Southeast-Asian ancestry or who have mild to severe hepatic impairment (Child-Pugh class A, B, C).
For patients aged 6 years and older who are of Asian ancestry OR who have mild to severe hepatic impairment, initiate PROMACTA at a reduced dose of 25 mg once daily
For patients aged 6 years and older who are of Asian ancestry WITH hepatic impairment, consider initiating at a reduced dose of 12.5 mg once daily
PROMACTA has a maximum dosage of 75 mg per day
No weekly injection required1
Oral Suspension
PROMACTA for oral suspension kits
- Available to order in boxes of 12.5-mg and 25-mg packets
- Request a Demonstration Kit through your local representative
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CsA, cyclosporine A; ITP, immune thrombocytopenia; IVIg, intravenous immunoglobulin; ULN, upper limit of normal.