Prescribing PROMACTA

EFFICACY & SAFETY
PROMACTA worked fast—rapid response seen as early as Week 11,2
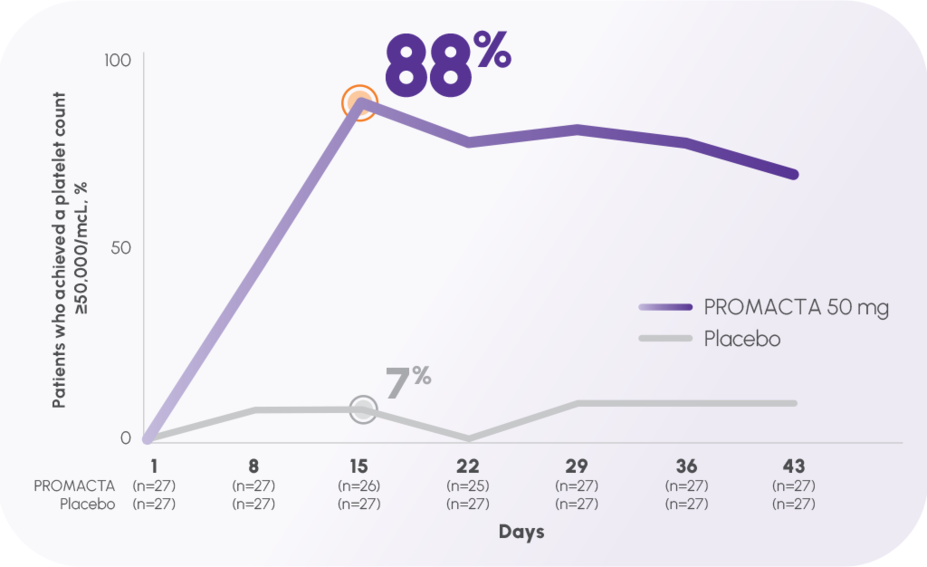
Nearly 9 of 10 patients responded at Week 2 (platelet count ≥50,000/mcL)1,2
In the TRA100773A pivotal trial:
The primary end point, response at Day 43, was 70% (19/27) with the 50-mg dose vs 11% (3/27) with placebo2,†
Response rate at Day 8 was 44% (12/27) vs 7% (2/27) with placebo2
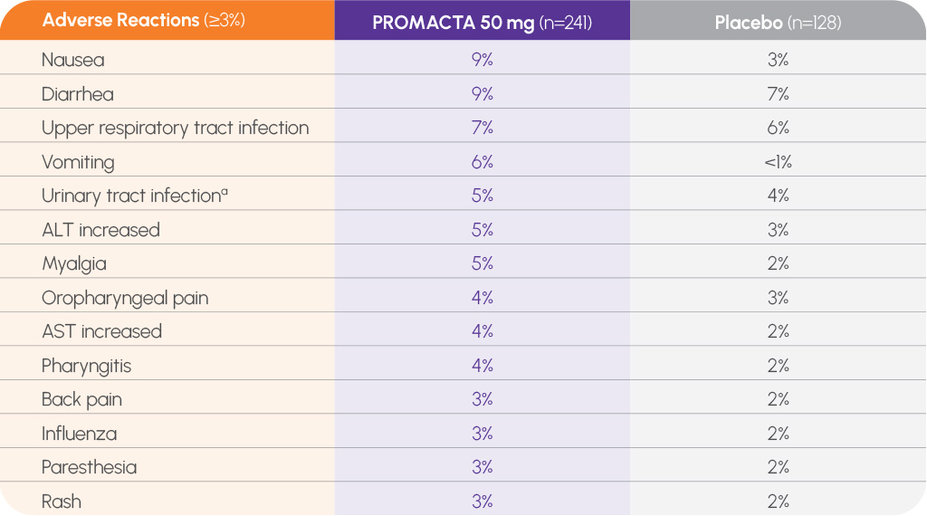
The incidence of adverse events was consistent across all groups while on treatment2
In the 3 placebo-controlled pivotal trials, serum liver test abnormalities (predominantly grade 2 or lower in severity) were reported in 11% and 7% of patients in the PROMACTA and placebo groups, respectively2
TRA100773A was a phase 2, 12-week, double-blind, placebo-controlled trial of 117 adult patients with previously treated ITP.1,2
Response was defined as a shift from baseline platelet count <30,000/mcL to ≥50,000/mcL at any time during the treatment period up to 42 days of dosing with 30 mg, 50 mg, or 75 mg of eltrombopag.1,2
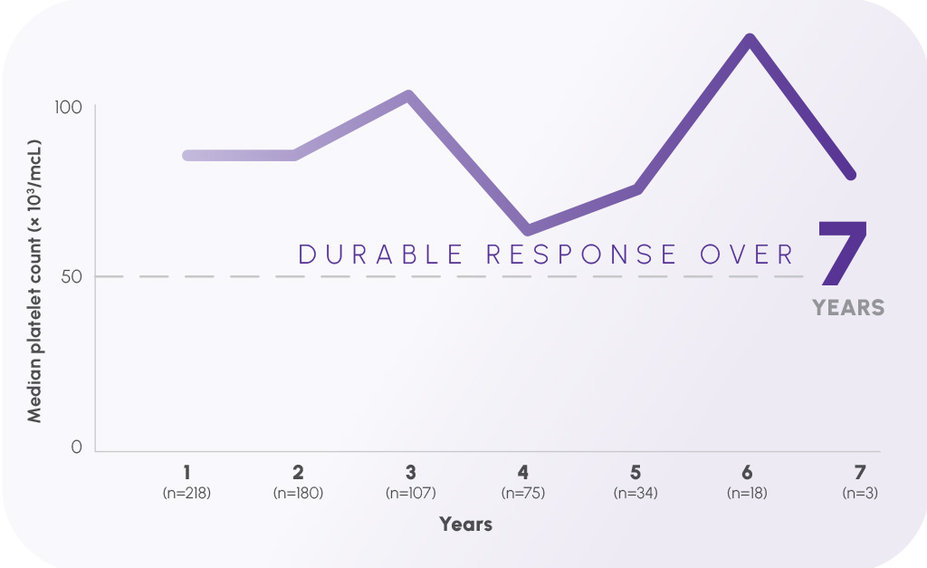
Up to 7 years of continuous response in the EXTEND trial—the longest results ever reported in persistent or chronic ITP1,3,5-7
Year over year, median platelet count remained ≥50,000/mcL1,3
In the EXTEND long-term trial:
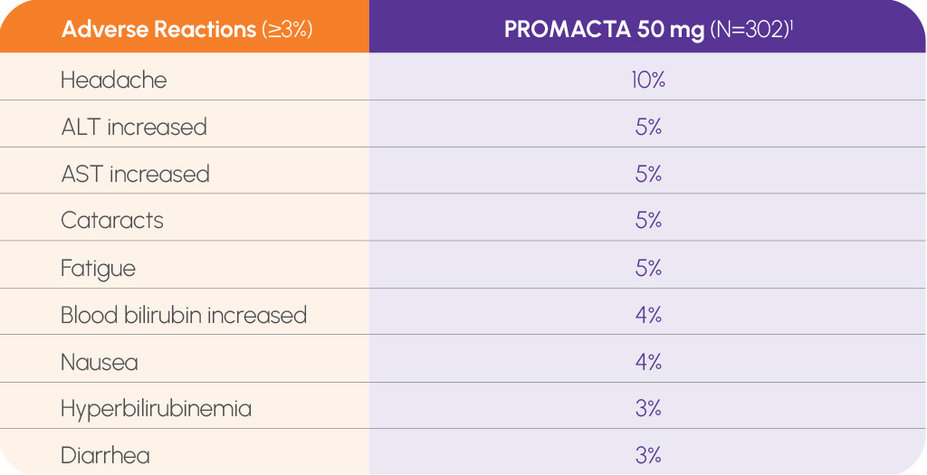
In the primary end point, no new adverse reactions were identified at 6 years of therapy1,3
The open-label EXTEND study evaluated the long-term safety and efficacy of eltrombopag in adults with ITP who had completed a previous eltrombopag study.1,3,8 The median baseline platelet count was 19,000/mcL prior to administration of PROMACTA.1,3 This study reviewed more than 8 years of continuous treatment.3,8 PROMACTA was administered to 302 patients in EXTEND; 218 completed 1 year, 180 completed 2 years, 107 completed 3 years, 75 completed 4 years, 34 completed 5 years, and 18 completed 6 years of therapy, with 3 patients evaluable at Year 7.1,3 Median platelet counts ≥50,000/mcL by Week 2 and at least that throughout.1,3,8 The primary end points were safety and tolerability.3
Consistent safety profile in short-term trials, including <3% risk of bleeding-related adverse reactions1
In pooled data from short-term trials, no occurrence ≥3% (and more frequent than with placebo) of contusion, epistaxis, gingival bleeding, or petechiae (n=241)1
a Includes urinary tract infection, cystitis, urinary tract infection bacterial, and bacteriuria.
In clinical trials, hemorrhage was the most common serious adverse reaction, and most hemorrhagic reactions followed discontinuation of PROMACTA. Other serious adverse reactions included thrombotic/thromboembolic complications1
In the long-term EXTEND trial, no new adverse reactions at 6 years1,3
In the long-term EXTEND study, no new safety signals despite increased duration of exposure to PROMACTA
302 patients were enrolled in the EXTEND trial; not all patients were evaluable at 6 years1,3
— Primary end points were safety and tolerability assessed via laboratory tests3
In the long-term EXTEND study, no increased risk of venous thromboembolism with PROMACTA3
No increase in incidence of previously identified safety concerns (thromboembolic events and hepatobiliary laboratory abnormalities)3
No clinically relevant increase in bone marrow reticulin or collagen fibers3
In clinical trials, discontinuation due to adverse events was reported in 7% of patients treated with PROMACTA 50 mg in TRA100773A (n=2/30), 4% in TRA100773B (n=3/76), 10% in RAISE (n=13/135), and 14% in EXTEND (n=42/302)1-3,9,10
DOSING & ADMINISTRATION
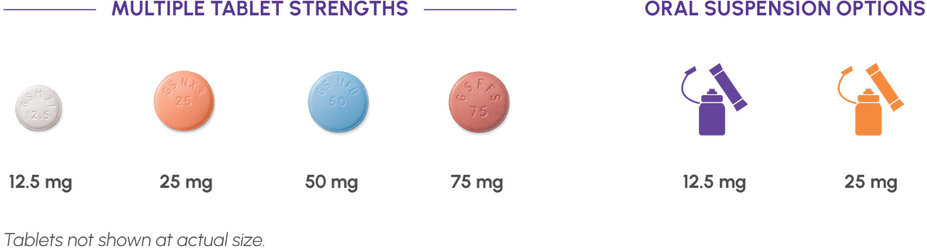
The convenience of once-daily oral dosing1
Patients can take PROMACTA without food or with food that is low in calcium (≤50 mg)1
PROMACTA should be taken at least 2 hours before or 4 hours after medications such as antacids and mineral supplements or foods high in calcium1
PROMACTA can be taken any time of day, at the same time each day1
No need for weekly office visits for injections, and with PROMACTA tablets, there is less unused medication to discard1,4
Dosing flexibility, even for patients who have difficulty swallowing a pill1
PROMACTA offers simplified titration to help maintain response over time1
a For patients of Asian ancestry, initiate at 25 mg/day. For patients with mild, moderate, or severe hepatic impairment, initiate at a reduced dose of 25 mg once daily; for patients of Asian ancestry with hepatic impairment, consider initiating at a reduced dose of 12.5 mg once daily.
b Do not exceed a dose of 75 mg daily. For patients taking 25 mg once daily, increase daily dose by 25 mg; for patients taking 12.5 mg once daily, increase dose to 25 mg daily before increasing the dose amount by 25 mg.
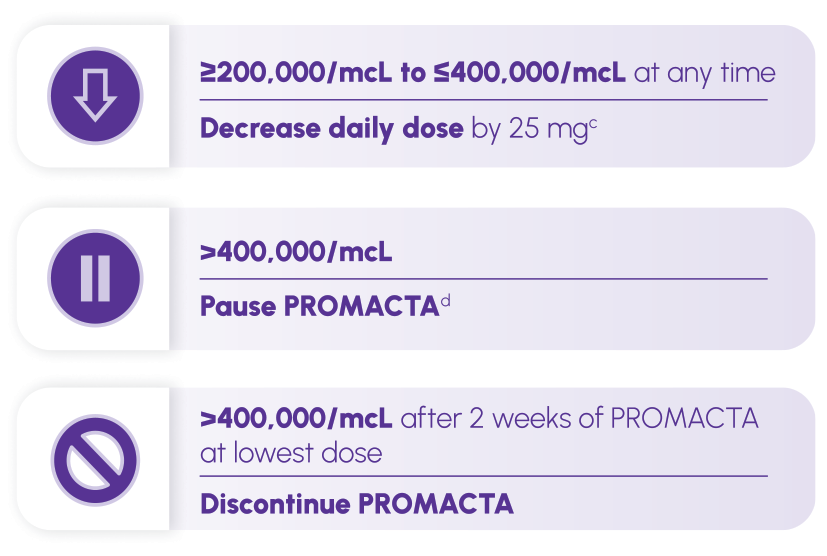
c Wait 2 weeks to assess the effects of this and any subsequent dose adjustments. For patients taking 25 mg once daily, decrease the dose to 12.5 mg once daily.
d Increase the frequency of platelet monitoring to twice weekly. Once the platelet count is <150,000/mcL, reinitiate therapy at a daily dose reduced by 25 mg. For patients taking 25 mg once daily, reinitiate therapy at a daily dose of 12.5 mg.
Monitor clinical hematology and liver tests regularly throughout therapy1
If platelet counts do not increase to a level sufficient to avoid clinically important bleeding after 4 weeks at 75 mg, discontinue therapy1
ALT, alanine aminotransferase; AST, aspartate aminotransferase; ITP, immune thrombocytopenia.