Prescribing PROMACTA

EFFICACY & SAFETY
IN FIRST-LINE TREATMENT OF SAA
Rapid response as early as 3 months2
OVERALL RESPONSE RATE AT 3 MONTHS (COHORT 3 + EXTENSION COHORT)2
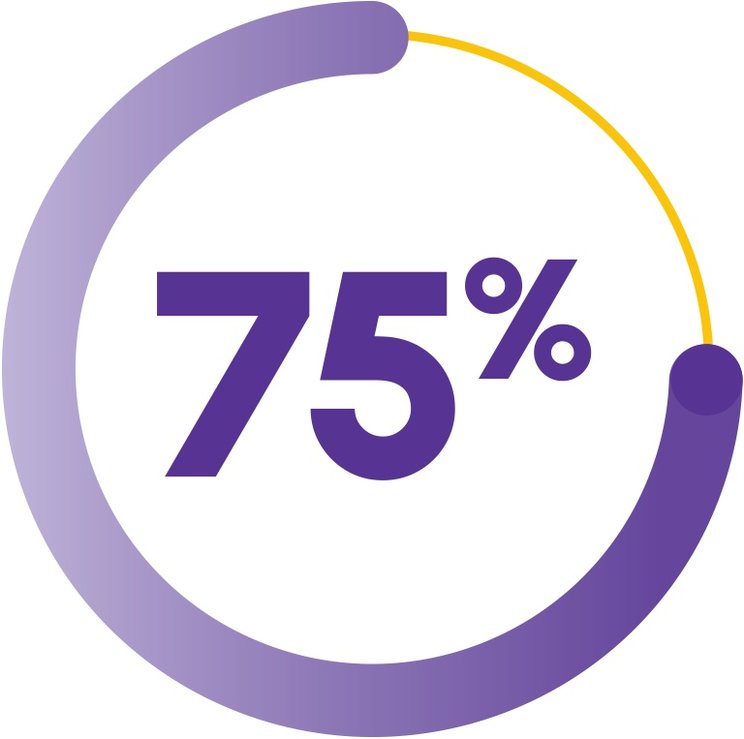
(n=66/88; 95% CI, 64.6-83.6)
3 of 4 patients
achieved a response with PROMACTA + IST2
Double their chances of a complete response by starting in 1L1-3
COMPLETE RESPONSE RATE AT 6 MONTHS (COHORT 3 + EXTENSION COHORT)1
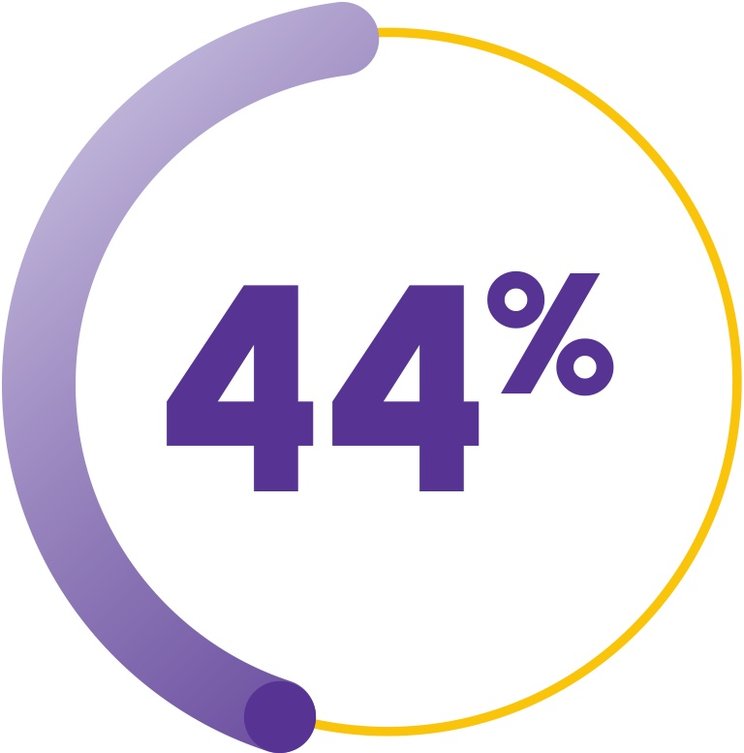
(n=38/87; 95% CI, 33-55)
>2x the historical rate
seen with standard IST alone (17%; n=17/102)1-3
Complete response was defined as hematologic parameters meeting all 3 of the following values on 2 consecutive serial blood count measurements at least 1 week apart: ANC >1000/mcL, platelet count >100,000/mcL, and Hb count >10g/dL1
OVERALL RESPONSE RATE AT 6 MONTHS (COHORT 3 + EXTENSION COHORT)1
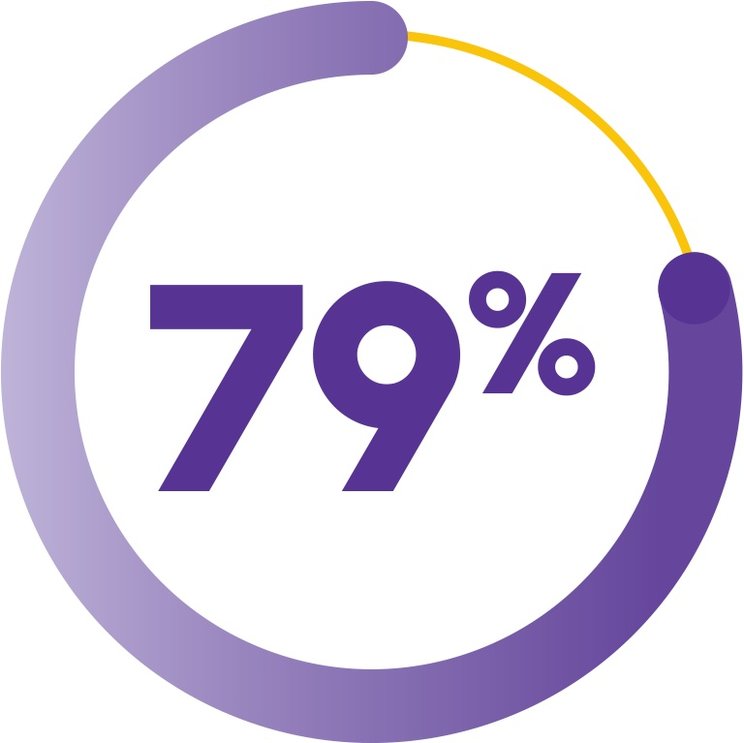
(n=69/87; 95% CI, 69-87)
vs 66% in the historical cohort receiving standard IST alone (n=67/102)1
Overall response rate was defined as the number of partial responses (blood counts no longer meeting the standard criteria for severe pancytopenia in SAA†) plus complete responses1
†Equivalent to 2 of the following values on 2 consecutive serial blood count measurements at least 1 week apart: ANC >500/mcL, platelet count >20,000/mcL, or reticulocyte count >60,000/mcL.1
SUSTAINED RESPONSE OFF TREATMENT DEMONSTRATED 18 MONTHS AFTER PATIENTS DISCONTINUED PROMACTA1,2
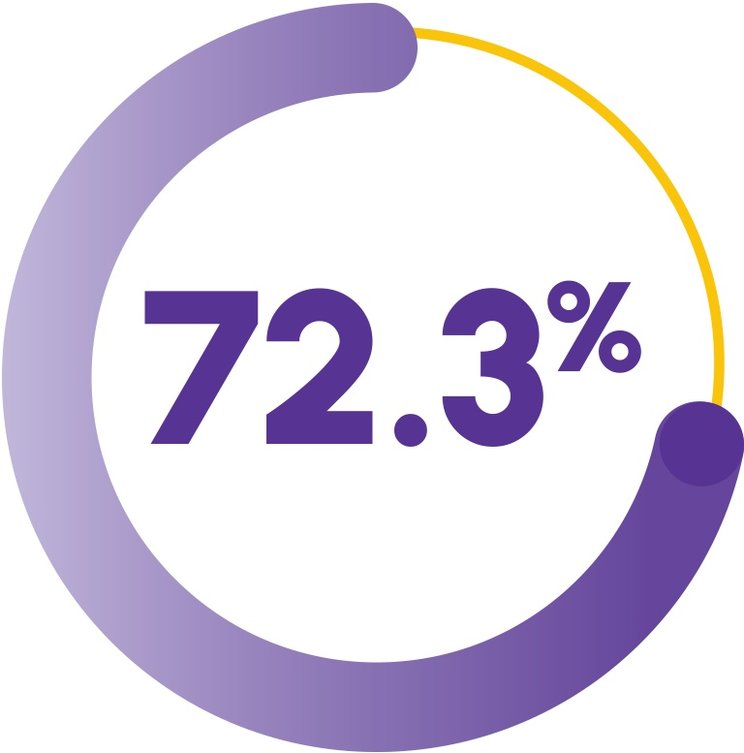
(n=67; 95% CI, 57.3-82.8)
For patients with complete or partial response to PROMACTA at 6 months (Cohort 3 + extension cohort), there was a 72.3% probability of maintaining that response 18 months postdiscontinuation2
The median duration of response–24.3 months–was consistent for patients achieving a complete response (n=46; 95% CI, 23.0-NE) or an overall response (n=70; 95% CI, 21.4-NE) at any time with PROMACTA + IST (Cohort 3 + extension cohort)1,2
Study Design
ETB115AUS01T was a single-arm, open-label, sequential cohort trial in patients 2 years of age or older (N=153).1 All cohorts received h-ATG Days 1 to 4 and CsA for 6 to 24 months.1,‡ Cohort 3 + extension cohort patients received PROMACTA Day 1 to 6 months (n=92).1,§ Sixty-six patients were ≥17 years of age and 26 patients were 2 to 16 years of age.1
‡Patients in all cohorts received a therapeutic dose of CsA Day 1 to 6 months.1 Following a protocol amendment, patients who responded at 6 months received a maintenance dose of CsA (2 mg/kg/day) for 18 months after discontinuation of PROMACTA.1,2 Fifteen responders in Cohort 2 (n=27/31) and 67 responders in Cohort 3 + extension cohort (n=69/87) received maintenance CsA.1,2
§Of these patients, 5 were not included in analyses of hematologic response at Month 6, as they had neither reached the 6-month assessment nor withdrawn earlier.1
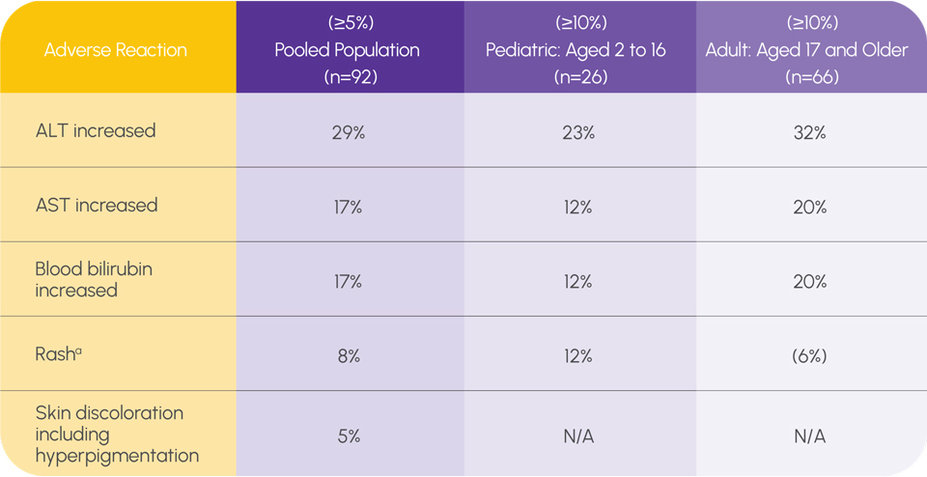
ADVERSE REACTIONS IN SAA IN FIRST LINE (COHORT 3 + EXTENSION COHORT)1
a The only serious adverse drug reactions experienced by ≥10% of pediatric patients were rash and upper respiratory tract infection (12% in patients age 2 to 16 years compared with 5% in patients 17 years of age and older).
Grade 3 and grade 4 liver function laboratory abnormalities were 15% and 2% for AST, 26% and 4% for ALT, and 12% and 1% for bilirubin, respectively
Patients had bone marrow aspirates evaluated for cytogenetic abnormalities. Seven patients in Cohort 3 + extension cohort had a new cytogenetic abnormality reported, of which 4 had the loss of chromosome 7
IN RELAPSED/REFRACTORY SAA
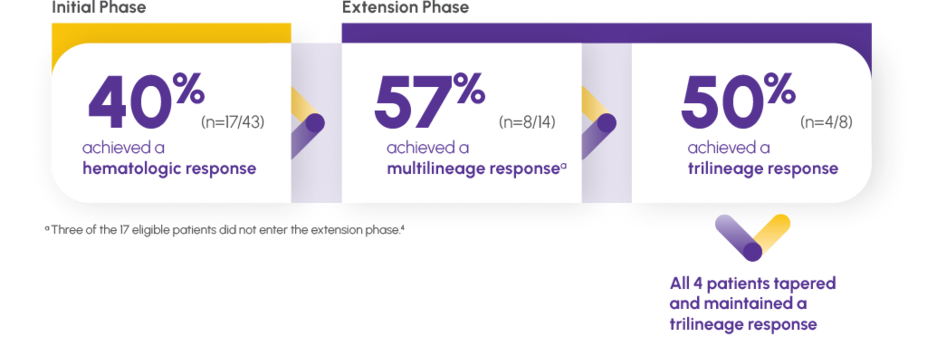
Make a trilineage difference: PROMACTA monotherapy provided a sustained multilineage response1
HEMATOPOIESIS MAINTAINED AFTER DISCONTINUATION1,4
A multilineage response is defined as an increase in production in ≥2 of the following hematologic markers: Platelets, white blood cells, and red blood cells4,5
Study Design
ELT112523 was a prospective, open-label, nonrandomized, single-arm, dose-modification, investigator-sponsored study conducted by the National Institutes of Health to assess the safety and efficacy of PROMACTA in patients with SAA and IST-refractory thrombocytopenia (N=43).1,4,5
The primary end point was hematologic response at Week 12 or Week 16, defined by meeting 1 or more of the following criteria1,5:
Platelet count increase to 20,000/mcL above baseline or stable platelet counts with transfusion independence for a minimum of 8 weeks
Hb count increase >1.5 g/dL for patients with pretreatment Hb count <9 g/dL or a reduction in ≥4 units of red blood cell transfusions for 8 consecutive weeks
ANC increase of 100% or >500/mcL
Patients who responded in the initial phase were eligible to continue therapy in an extension phase.1,4
Patients had a median age of 45 years and either had an insufficient response to at least 1 prior course of IST or were relapsed/refractory and had responded to at least 1 prior cycle of IST but were refractory to the most recent course of IST.1,5
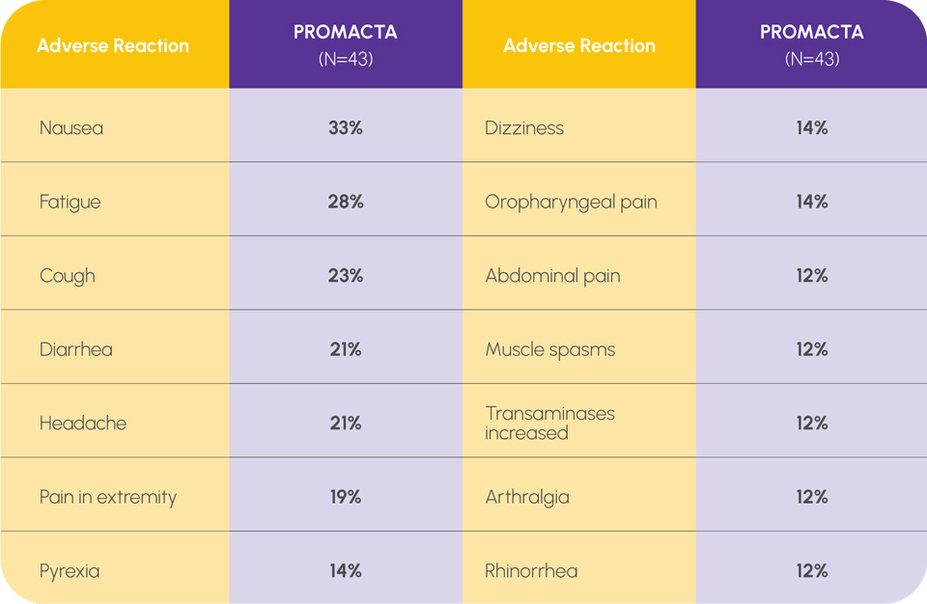
ADVERSE REACTIONS IN THE RELAPSED/REFRACTORY POPULATION (≥10%)1
If new cytogenetic abnormalities are observed, consider discontinuation of PROMACTA
Patients had bone marrow aspirates evaluated for cytogenetic abnormalities
Eight patients had a new cytogenetic abnormality reported while on therapy, including 5 patients who had complex changes in chromosome 7
DOSING & ADMINISTRATION
FOR ALL PROMACTA PATIENTS
PROMACTA can be taken without food or with food low in calcium (≤50 mg)1
PROMACTA should be taken at least 2 hours before or 4 hours after medications such as antacids and mineral supplements or foods high in calcium1
PROMACTA can be taken any time of day, at the same time each day1
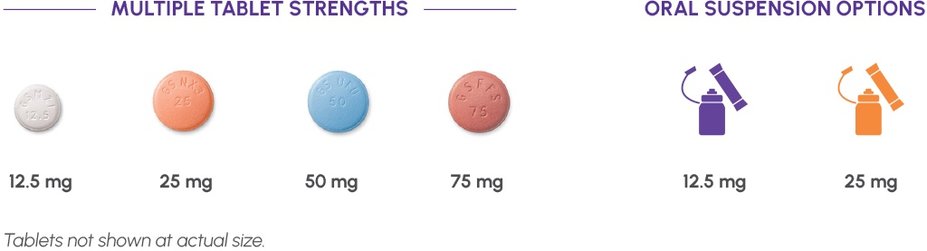
Available Formulations
Dosing flexibility, even for patients who have difficulty swallowing a pill1
IN FIRST-LINE TREATMENT OF SAA
Initiate PROMACTA concurrently with h-ATG + CsA1

For patients with SAA who are of Asian ancestry or those with mild, moderate, or severe hepatic impairment (Child-Pugh Class A, B, C), PROMACTA should be initiated at a dose of 75 mg once daily for 6 months.

For patients with SAA who are of Asian ancestry or those with mild, moderate, or severe hepatic impairment (Child-Pugh Class A, B, C), PROMACTA should be initiated at a dose of 37.5 mg once daily for 6 months.

For patients with SAA who are of Asian ancestry or those with mild, moderate, or severe hepatic impairment (Child-Pugh Class A, B, C), PROMACTA should be initiated at a dose of 1.25 mg/kg once daily for 6 months.
Do not exceed the recommended initial dose of PROMACTA
A single treatment course of h-ATG should be administered intravenously at a dose of 40 mg/kg/day for 4 consecutive days starting Day 11
CsA should be administered at a dose of 6 mg/kg/day (12 mg/kg/day for children under 12 years of age) from Day 1 for 6 months1
FOR PATIENTS WITH RELAPSED/REFRACTORY SAA
Initiate as monotherapy1

Adjust the dose to maintain a platelet count ≥50,000/mcL. Do not exceed 150 mg per day.
For patients with SAA who are of Asian ancestry or have mild, moderate, or severe hepatic impairment, PROMACTA should be initiated at a reduced dose of 25 mg once daily.
1L, first line; ALT, alanine aminotransferase; ANC, absolute neutrophil count; AST, aspartate aminotransferase; CsA, cyclosporine A; h-ATG, horse antithymocyte globulin; Hb, hemoglobin; IST, immunosuppressive therapy; NE, not estimable; SAA, severe aplastic anemia.